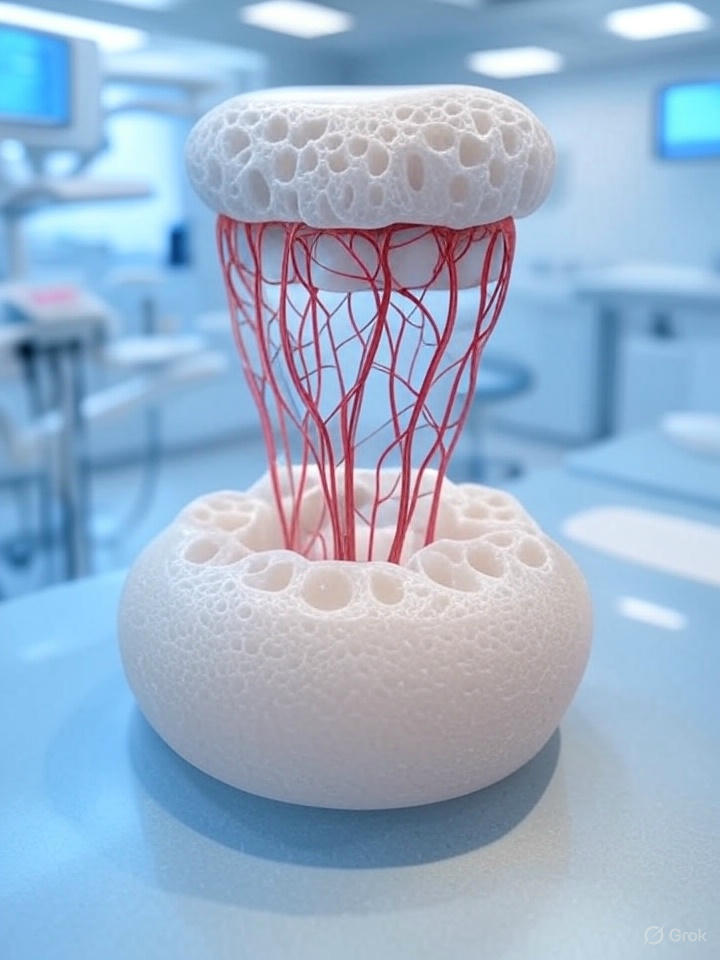
In this DentistryUnited news analysis, we examine a groundbreaking development in restorative dentistry from Tufts University’s School of Dental Medicine, where a novel neurointegrative dental implant promises to redefine tooth replacement. Traditional titanium-based implants, reliant on osseointegration, lack the sensory feedback provided by the periodontal ligament’s neurovascular network, leading to compromised masticatory function and patient comfort. Led by Professor Jake Jinkun Chen and former Ph.D. student Wei Qiao, this innovative implant leverages regenerative biomaterials to restore soft-tissue and neural connectivity, offering a biomimetic alternative (Tufts University, 2025). This report evaluates the implant’s design, preclinical findings, and implications for clinical practice, situating it within the evolving landscape of regenerative dental therapeutics.
Key Findings
The Tufts implant utilizes a biodegradable nanofiber scaffold infused with dental pulp stem cells (DPSCs) and fibroblast growth factor 2 (FGF-2), a neurogenic protein. Electrical stimulation enhances nerve regeneration, enabling integration with gingival tissue and synaptic connectivity with the trigeminal nerve (Tufts University, 2025). Unlike conventional implants, which require invasive bone drilling, this system occupies the alveolar socket, promoting in situ tissue regeneration without osseous fixation.
Preclinical rodent studies demonstrate promising nerve integration, with implants exhibiting pressure sensitivity comparable to natural dentition. Electromyographic and behavioral assessments suggest functional restoration, though specific timelines remain undisclosed (Tufts University, 2025). The implant’s design reduces peri-implantitis risk and biomechanical stress on the alveolar ridge, addressing common complications in implantology.
Implications for Restorative Dentistry
The neurointegrative implant offers a paradigm shift in restorative dentistry by restoring pressure sensitivity, potentially enhancing masticatory efficiency and patient quality of life. Its minimally invasive approach aligns with regenerative dentistry’s focus on biomimetic solutions, leveraging stem cell-mediated tissue engineering to recapitulate native tissue architecture. The global dental soft-tissue regeneration market, valued at USD 0.38 billion in 2025, is poised for growth as such innovations address unmet clinical needs (Decisions in Dentistry, 2025).
Beyond dentistry, the implant’s neurointegrative framework holds potential for orthopedic applications, such as nerve-integrated prostheses, as suggested by Chen (Tufts University, 2025). The biodegradable scaffold mitigates foreign body reactions, promoting host tissue integration and reducing complication rates compared to titanium implants. This technology could decrease reliance on invasive procedures, improving clinical outcomes and patient satisfaction.
Critical Evaluation
While preclinical results are encouraging, significant translational challenges remain. Rodent models, though valuable, exhibit physiological differences that limit extrapolation to human applications. Planned trials in larger animal models, such as porcines, will be critical to assess scalability and long-term biocompatibility (Tufts University, 2025). Human clinical trials, necessary for regulatory approval, must evaluate implant durability under occlusal loads and address potential immunogenicity from allogeneic DPSCs.
The reliance on stem cells raises logistical and economic concerns. Autologous harvesting is resource-intensive, while allogeneic approaches risk immune rejection. The cost-effectiveness of stem cell-based therapies in public health systems, such as the NHS, remains uncertain, particularly given the high cost of current regenerative treatments (News Medical, 2025). Comparative studies with titanium implants and emerging regenerative therapies, such as tooth-regrowing biologics at Kyoto University, are needed to establish clinical and economic viability.
The implant’s alignment with regenerative trends, including bioengineered teeth by Tufts’ Professor Pamela Yelick, underscores its potential to reshape restorative dentistry (Newswise, 2025). However, patient-specific factors, such as alveolar bone quality or comorbidities, may influence outcomes. Long-term studies are essential to confirm neural functionality and implant stability across diverse cohorts.
Moving Forward…
The Tufts neurointegrative dental implant, developed by Chen and Qiao, represents a significant advancement in restorative dentistry, offering a biomimetic alternative to conventional implants. By integrating stem cells, neurogenic factors, and electrical stimulation, it promises to restore pressure sensitivity with minimal invasiveness. While preclinical data are promising, rigorous translational research is required to address regulatory, technical, and economic barriers. If successful, this technology could redefine implantology and inspire broader regenerative innovations.
References
- Tufts University (2025) ‘What if dental implants could feel more like your real teeth?’, Tufts Now, 11 June. Available at: https://now.tufts.edu/2025/06/11/what-if-dental-implants-could-feel-more-your-real-teeth (Accessed: